With manganese dioxide as catalyst, activated carbon as catalyst support, teflon as the binder of gas diffusion electrode is a three-phase reaction groove electrode interface. Mainly used in alkaline metal air batteries, for the reduction of oxygen to provide a reaction site. With the increased demand for high-power power battery, the nature of the gas diffusion electrode was also hit by the unprecedented challenges. Both require the use of gas diffusion electrode low cost, but also requires the electrode has a higher output power. Since the use of manganese dioxide as the oxygen reduction catalyst, the use of Pt, Au and other precious metals as a catalyst when the electrode cost.
In determining the catalyst, the main factor determining the performance of the gas diffusion electrode is the microstructure of the electrode. The ideal gas diffusion electrode structure on the one hand must have good gas permeability to meet the high power of the electrode when the gas reactant supply; On the other hand have a good porous system, and can form enough three-phase interface, improve the gas diffusion electrode chemical energy into electrical energy capacity. By increasing the effective porosity of gas diffusion electrode structure to increase electrochemical reaction sites become one of the method of improving electrode performance. Adding a certain amount of pore-forming agent in the electrode can increase the permeability of the electrode to the reaction gas and reduce the concentration polarization. The formation of the porous system of the gas diffusion electrode is related to the structure of the catalyst support used, Carbon nanotubes as a catalyst carrier can make the gas diffusion electrode to form a unique network structure, greatly reducing the transmission of reactive gas barriers, while increasing the electrode electrochemical reaction activation point. In addition, fiber, activated carbon, nickel fiber mixture, etc. through the sintering to form a unique highly efficient porous structure, and the thickness does not exceed 2/3 of the conventional electrode. This electrode not only has good electronic conductivity, but also has a high efficiency of electrochemical performance.
The structure of the gas diffusion electrode is divided into a gas diffusion layer and a reactive layer (catalyst layer). The gas diffusion layer mainly exercises the reaction gas transmission function, and the reactive layer is mainly the place where the electrochemical reaction is provided. teflon emulsion as adhesive of gas diffusion electrode, On the one hand with the function of adhesive electrode material, on the other hand with the microstructure of the electrode itself is closely linked. In this paper, the pretreatment of teflon is added to the production process of gas diffusion electrode, the influence of the properties of the diffusion electrode on the diffusion electrode and the microstructure characteristic parameters of the gas diffusion electrode were discussed. The effect of the teflon powder on the performance of the gas diffusion electrode was discussed.
The experiment
The manufacture of gas diffusion electrode
Production process of gas diffusion layer. The acetylene black, graphite, activated carbon by 6: 3: 4 mass ratio with 20000 r·min-1 mixer evenly mixed, a certain amount of 30% (w, mass fraction) of teflon emulsion is mixed with the corresponding amount of ethanol (anhydrous ethanol, analytically pure), and than the two mixtures were uniformly blended and applied to the side of the foamed nickel, bake in an oven at 150 ℃ for 30 min.
Production process of catalytic layer. First, acetylene black, graphite, activated carbon, manganese dioxide by 2: 7: 9: 54 mass ratio with the same speed mixer evenly mixed, then a certain amount of 30% teflon latex mixed with appropriate amount of ethanol, and than the two mixtures were uniformly blended and applied to the side of the foamed nickel, bake in an oven at 150 ℃ for 30 min.
The baked electrode is naturally cooled to room temperature, A 0.1 mm thick teflon film was attached to the gas diffusion layer side to prevent the electrolyte leakage. Finally, the electrode is rolled and the thickness is about 0.5 mm.
Gas diffusion electrode performance testing
Gas diffusion electrode effective area is 70 cm2, with zinc electrode assembled into zinc air battery, 7 mol·L-1 KOH solution as the electrolyte, at room temperature and atmospheric pressure in the air discharge performance test. Zinc-air batteries in the first 30 minutes before the start of work, zinc electrode can be considered no change. It is generally believed that the overpotential of the zinc air battery is mainly produced by the cathode, In the experiment with calomel reference electrode measurements that, when the current density in 200, mA at 2 cm – produced by zinc electrode overpotential was only about 0.02 V, compared with the overpotential of gas diffusion electrode is a very small amount, it can be ignored. When increasing battery working current density of overpotential can approximate thought is caused by a gas diffusion electrode. Compared with zinc air battery voltage value under same working current density can determine gas diffusion electrode discharge performance. Each kind of experiment to repeat more than three times at least, to ensure the reliability of the experimental results.
Through automatic microporous physical and chemical adsorption instrument (American Microm – eritics ASAP2020M + C) to a single point of gas diffusion electrode BET specific surface area, Langmuir specific surface area, pore distribution, and total pore volume and area of the test. By scanning electron microscope (SEM, American FEI, Sirion200) observe apparent morphology of electrode.
The pretreatment of ethanol
Teflon film teflon membrane immersed in ethanol solution, and then at 150 ℃ conditions, the ethanol completely volatile, observed throughout the process of teflon membrane morphology changes.
The results and discussion
Discharge performance of gas diffusion electrode
Only the diffusion layer side teflon emulsion is pretreated
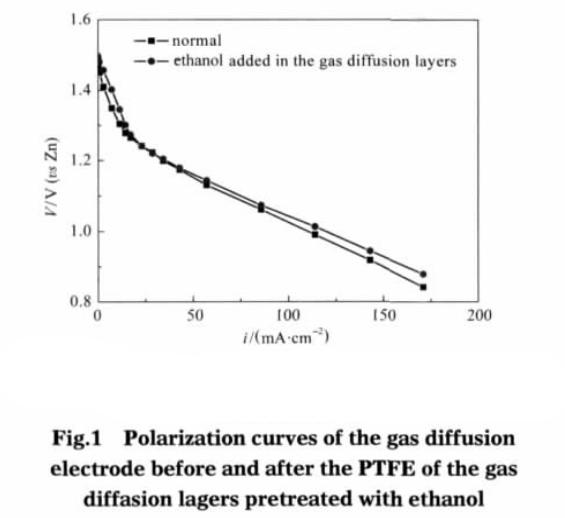
From the battery discharge results (as shown in figure 1), only the gas diffusion layer of teflon adhesive emulsion after pretreatment, other things being equal, compared with the adhesive without pretreatment of electrode, the current density is 110 cm, mA – 2, gas diffusion electrode polarization overpotential reduced 23 mV. This indicates that the gas diffusion layer adhesive after ethanol pretreatment can improve the performance of the electrode, but the magnitude is very small.
Only the catalyst layer side teflon emulsion was pretreated
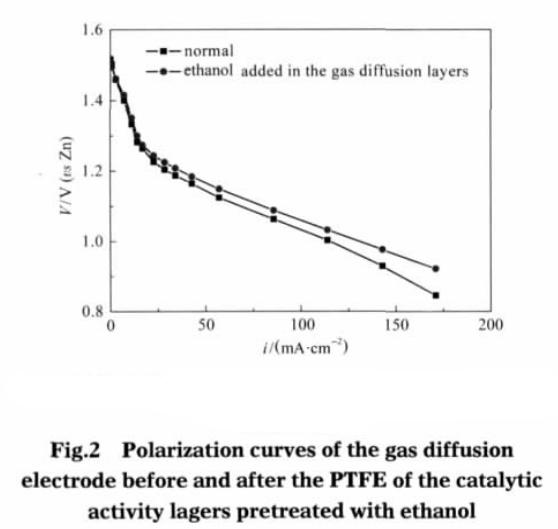
Fig.2 shows the polarization curves of the catalytic layer teflon before and after ethanol pretreatment. As can be seen from Fig. 2, only the catalytic layer of adhesive teflon emulsion after ethanol pretreatment it can also significantly improve the performance of gas diffusion electrode. At relatively low current density (less than 50 mA · cm-2), the adhesive of the catalyst layer was not significantly different from the pretreated one. When the working current density exceeds 100 mA · cm-2, the polarization overpotential of the pretreated gas diffusion electrode of teflon emulsion is obviously lower than that without pretreatment. When the working current density is about 140 mA · cm-2, compared with the conventional electrode, the catalytic layer of teflon emulsion after pretreatment of gas diffusion electrode polarization overpotential was reduced 47 mV. This shows that the relatively high current density at work, the catalytic layer adhesive after ethanol pretreatment can significantly improve the performance of gas diffusion electrode.
Changes of teflon Membrane Before and After Pretreatment with Ethanol
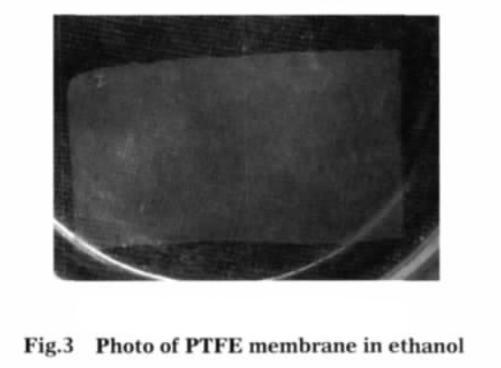
Fig.3 is a photograph of a teflon film in ethanol. As shown in fig.3, the white teflon film is translucent in ethanol and can be completely immersed in ethanol, hydrophobic teflon membranes are hydrophilic in nature. After 150 ° C baking to completely evaporate ethanol, teflon film change into the original white, and was curved (see Figure 4). After treated with ethanol, teflon membrane has a certain degree of contraction.

Discussion
Teflon film in ethanol will be water swelling, and the ethanol is completely evaporated, the teflon film will shrink and resume, but the chemical properties do not change. teflon membrane and teflon emulsion is the same kind of material of two different forms, with the same chemical properties. teflon emulsion as adhesive will swell when mixed with ethanol, and after the powdery electrode material is uniformly blended, the teflon emulsion and the electrode material form micelles. The micelles and micelles are bonded to each other to form a catalytic layer and a gas diffusion layer of the gas diffusion electrode, and then at the condition of 150 ℃ drying, ethanol and water evaporation, teflon shrinkage, to a certain extent, the gas diffusion electrode played a role in making holes. Microscopic observation on electrode proved, adhesive after pretreatment, far more than the pore structure of the gas diffusion electrode without pretreatment of electrode. Comparison of gas diffusion electrode SEM photos (see figure 5 and figure 6) can be concluded that the adhesive without pretreatment of gas diffusion electrode apparent morphology show flat shape, pore and fissure is not obvious. After pretreatment, the surface of the gas diffusion electrode catalytic layer is almost covered with fissures and pores, and particles with different sizes of particles appear.
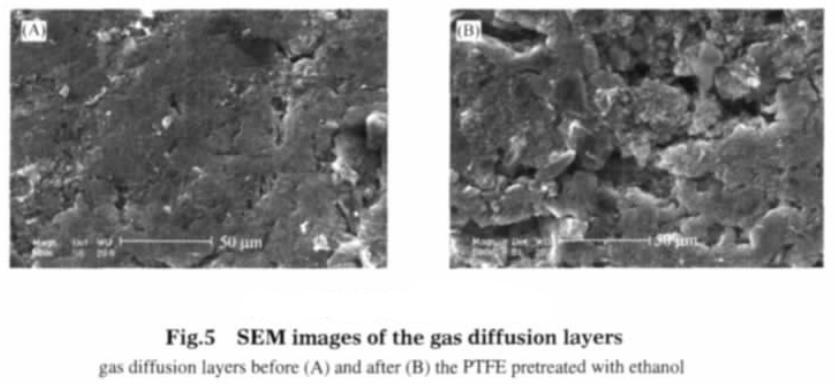
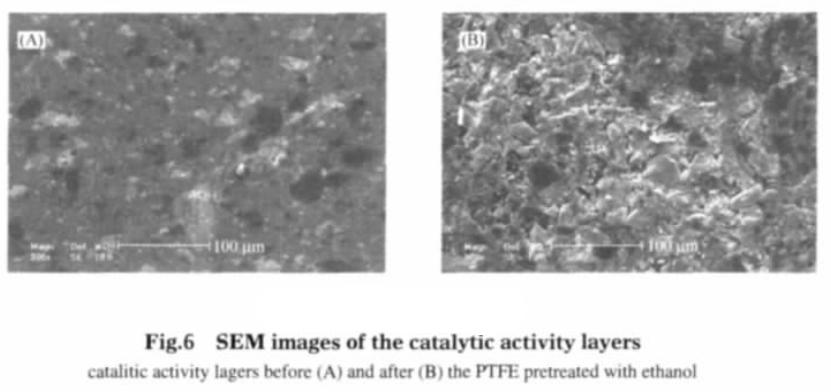
Figure 7 is BET test results, the straight line is based on p/(Q (p0 – p)) as the ordinate and (p/p0) as abscissa. The BET equation can calculate the gas diffusion electrode single saturated adsorption amount, Vm and Vm characterization of specific surface area:
![]()
Type p as adsorbate in partial pressure; P0 as the adsorbent of saturated vapor pressure; Q as actual amount adsorbed sample; The Vm as single sample saturated adsorption capacity; C as adsorption capacity related to the sample of constant, this type of value is 122.846875.
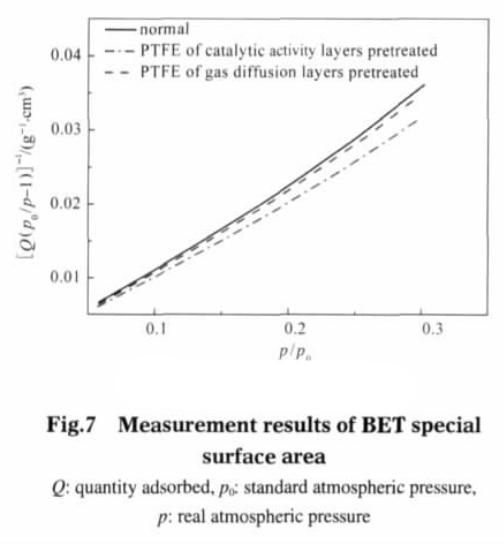
The calculated results show that the specific surface area of the electrode catalytic layer is 41.6962 m2 · g-1 after pretreatment; The diffusion layer was pretreated followed by 37.6578 m2 g-1; Untreated electrodes, specific surface area is 36.6563 m2 g – 1.. Figure 8 shows the Langmuir test results, with p/Q as the ordinate, p as the abscissa. By the Langmuir isotherm adsorption model equation calculates the Vm (m2 g – 1).
![]()
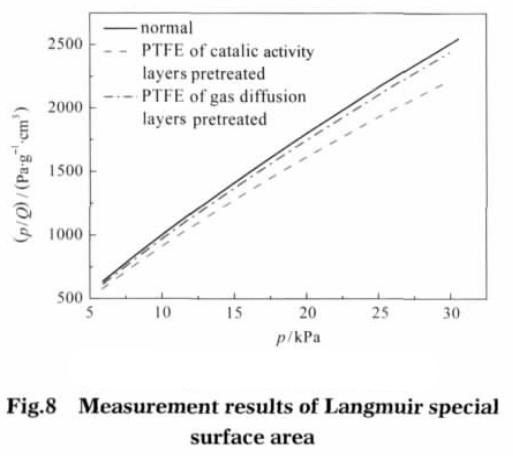
Value type b is adsorption coefficient, in this value is 3.453 x 10-4 Pa-1. After the pretreatment of the catalytic layer, the specific surface area of the electrode is the largest, which is 63.8552 m2 · g-1; after preprocessing the electrode diffusion layer, specific surface area is 57.4402 m2 g – 1; without pretreatment of electrode, specific surface area is 56.0801 m2 g – 1. This shows that the catalytic layer adhesive after ethanol pretreatment can significantly increase the specific surface of electrode, gas diffusion layer of specific surface area increased after pretreatment is not very obvious. The different diameter and contribution to the electrode surface as shown in figure 9, whether electrode after ethanol pretreatment, the aperture between 1-2 nm porous provided almost no change, specific surface area can be speculated that the number of microporous aperture is mainly related to the production of electrode materials used. The catalytic layer adhesive after alcohol pretreatment, the micropores with pore size between 2.5 and 10.0 nm increase significantly, and the specific surface area of micropores in pore size increases. The gas diffusion layer has no obvious change after pretreatment, performance compared with conventional electrodes in the aperture range of electrode surface have no obvious change. This shows that the binder after ethanol pretreatment, can increase the catalytic pore size between 2.5-10.0 nm between the number of micropores.
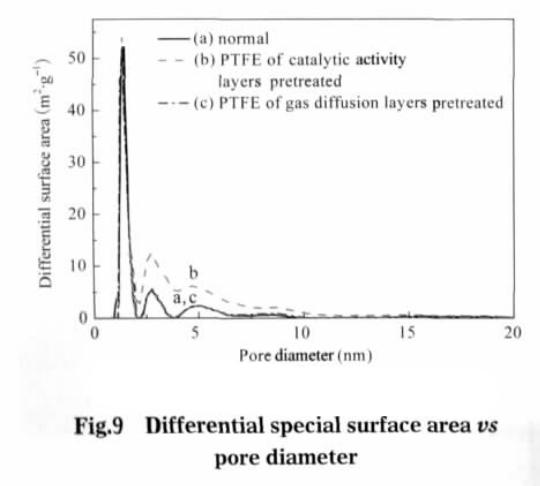
The main function of the gas diffusion layer is to adsorb and transfer the reaction gas. At the same time, the electrolyte is transferred from the catalytic layer to the diffusion layer, and a new electrochemical reaction site can be formed in the diffusion layer. Increasing the specific surface area of gas diffusion layer , on the one hand, reduce the reaction of gas transmission, on the other hand also can form more new reaction activation points, improving electrode performance. But the gas diffusion layer is thin, the thickness is about 1/10 the thickness of electrode, the electrode overall contribution to the surface is not big. So the gas diffusion layer adhesive after pretreatment, the specific surface area increases is not very obvious, showing a slight improvement in electrode discharge performance (see Fig.1).
The main function of the gas diffusion electrode catalytic layer is to provide a reaction site for electrochemical reactions (also known as reaction activation points). Generally, the number of effective reaction sites is used to define the ability of the gas diffusion electrode to convert chemical energy into electrical energy. The more effective reaction sites, the greater the total gas diffusion electrode current contributes. So in the same condition of discharge voltage, effective place more gas diffusion electrode output current. The purpose of the catalytic layer of porous gas diffusion electrode is for more effective reaction activation point. teflon adhesive emulsion after pretreatment, catalyst layers obviously increase the specific surface area and porosity, effective reaction sites will also be increased accordingly. So under the condition of high current density, polarization overpotential is lower than conventional gas diffusion electrode in the same conditions (see Fig. 2).
The best content of ethanol
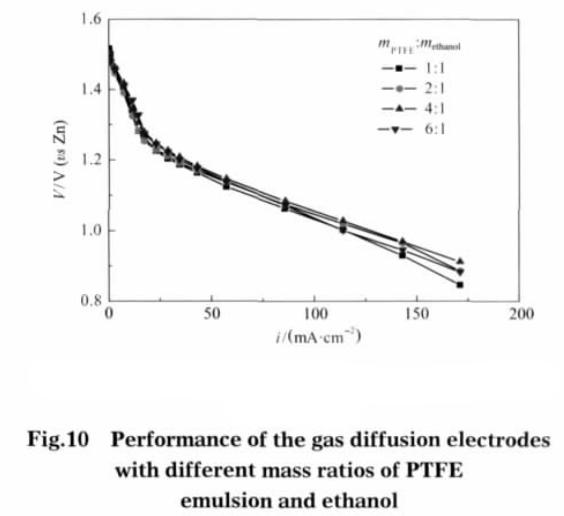
As ethanol is first mixed with teflon emulsio, there should be an optimum ratio between the amount of teflon emulsion and the amount of ethanol added. Figure 10 is ethanol electrode discharge performance of teflon emulsion with different ratio. It can be seen from figure 10, teflon emulsion with ethanol ratio of 4:1, the quality of the work in the same current density of gas diffusion electrode polarization potential minimum. The reason is the amount of ethanol added is too low, teflon expansion is not sufficient, the gas diffusion electrode pore formation did not achieve the best condition; Ethanol added too much, may lead to the initial bonding between the electrode powder material is not borne by the teflon, but by the ethanol, after high temperature drying, the original particles temporarily bonded by ethanol will relax, or even fall off, to the opposite effect.
Conclusion
Teflon in ethanol can be water swelling, until the ethanol and water completely evaporate teflon will shrink. The teflon emulsion is pretreated when the gas diffusion electrode is made to increase the electrode pore structure and specific surface area. The catalytic layer after pretreatment, specific surface area and pore structure increase more obviously, the catalytic layer increased pore structure can increase the gas diffusion electrode electrochemical reaction effectively. When under the condition of high current density, electrode polarization potential is relatively small; the gas diffusion electrode performance improvement is relatively significant. The increase of the specific surface area and pore structure of the electrode after the pretreatment of the gas diffusion layer is not obvious, and the improvement of the electrode performance is also limited. When the ratio of the mass of the teflon emulsion to the ethanol is 4: 1, the electrode performance is the best.
If you need more information about our products, please contact us: infocorefrp@gmail.com ,our engineers will answer you and provide free samples.